大肠杆菌载体 E.coli Vector 大肠杆菌宿主菌株 E.coli 细菌广宿主载体 bacateria broad range host vector 链霉菌载体及菌株 Streptomyces 芽孢杆菌载体 Bacillus vector 芽孢杆菌宿主菌株 乳酸菌载体 lactic acid bacteria vector 乳酸菌宿主菌株 lactic acid bacteria strain 细菌基因敲除载体 毕赤酵母载体 毕赤酵母宿主菌株 酿酒酵母载体 酿酒酵母宿主菌株 丝状真菌载体 mold/fungi vector 乳酸克鲁维酵母载体 酵母真菌基因敲除基因编辑载体 植物细胞载体 plant cell vector 农杆菌菌株Agrobacterium tumefaciens strain 植物细胞基因敲除载体 plant cell 哺乳动物细胞载体 哺乳动物细胞荧光载体 荧光素酶报告基因载体 哺乳动物细胞基因敲除基因编辑载体 杂交系统 慢病毒载体 腺病毒载体 逆转录病毒载体 杆状病毒表达载体 基因干扰 RNAi载体 基因/cDNA/ORF 转座子质粒系统 transposon 金黄色葡萄球菌载体 staphylococcus aureus 假单胞菌载体 噬菌体 phage 不动杆菌载体 双岐杆菌载体 藻类表达载体 链球菌载体 厌氧菌载体 基因治疗载体 大肠杆菌基因突变体菌株 细菌荧光质粒 白色念珠菌载体 体外转录载体 谷氨酸棒杆菌载体 酿酒酵母基因突变体菌株 线虫载体 斑马鱼载体 Zebra fish 果蝇,昆虫载体Drosophila 鱼类细胞载体 fish cell 分支杆菌载体 克雷伯菌 枯草芽孢杆菌基因缺失突变株 基因ORF 金黄色葡萄球菌基因敲除突变株 肺炎克雷伯菌基因敲除突变株
| 产品编号 | 产品名称 |
| 乳酸乳球菌食品级载体pNZ8122 |
pNZ8122乳酸菌食品级表达载体
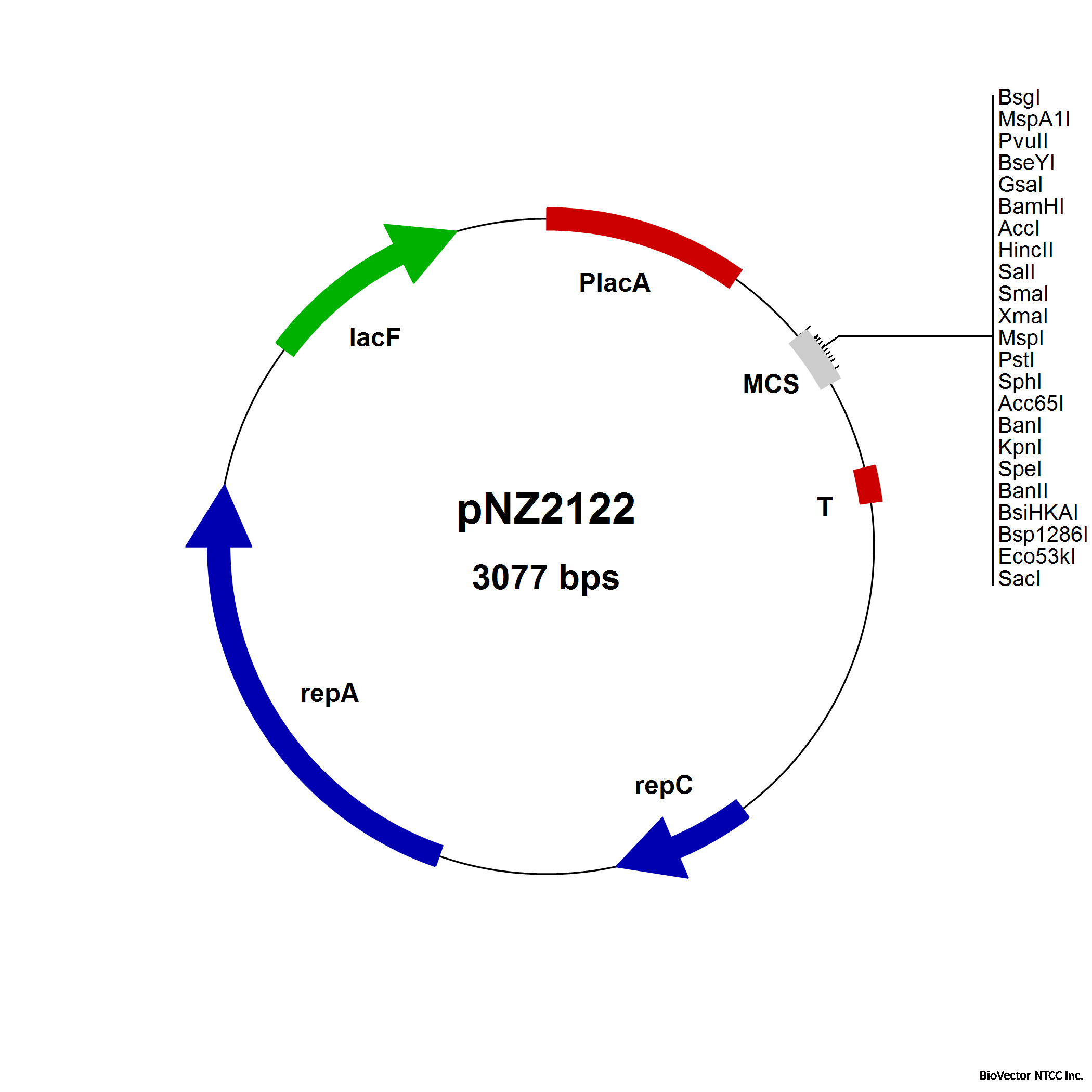
pNZ2122 plasmid
Food grade vector for constitutive gene expression under control of the lacA promoter for
L. lactis NZ3000. For transcriptional fusions. LacF-based selection. (Platteeuw et al.,1996)
Host strains
Lactococcus lactis subsp. cremoris MG1363
Plasmid-free progeny of the dairy strain NCDO712. Most widely used host strain for
cloning and gene expression in L. lactis (Gasson, 1983).
Lactococcus lactis NZ3000
Standard strain for food grade selection based upon the ability to grow on lactose. The
lactose operon, that is generally present on plasmids, has been integrated into the
chromosome and the lacF gene was deleted. Deletion of the lacF gene makes this strain
unable to grow on lactose unless lacF is provided on a plasmid (van Alen-Boerrigter, I. J.,
and W. M. de Vos, unpublished data; de Ruyter et al., 1996).
Strains Plasmids Medium
Lactococcus lactis MG1363 non-food grade M17 + 0.5% glucose + 10 μg/ml chloramphenicol
Lactococcus lactis NZ3000 food grade see 3.2 & 3.6
2.2 Plasmids
All plasmids are based on the pSH71 rolling circle replicon (de Vos, 1987).
pNZ124 General broad host range cloning vector with multiple cloning site for L. lactis and other
lactic acid bacteria. Chloramphenicol selection. (Platteeuw et al., 1994)
pNZ2105 General food grade cloning vector with multiple cloning site for L. lactis NZ3000. LacFbased
selection. (Platteeuw et al., 1996)
pNZ2103 Broad host range vector for constitutive gene expression under control of the lacA
promoter. For transcriptional fusion. Chloramphenicol selection. (Platteeuw et al., 1996)
pNZ7021 Broad host range vector for constitutive gene expression under control of the strong pepN
promoter. For transcriptional fusion. Chloramphenicol selection. (Wegkamp et al., 2007)
pNZ2122 Food grade vector for constitutive gene expression under control of the lacA promoter for
L. lactis NZ3000. For transcriptional fusions. LacF-based selection. (Platteeuw et al.,1996)
pNZ2123 Food grade vector. Identical to pNZ2122, but with inverted multiple cloning site (MCS).
LacF-based selection. (Platteeuw et al., 1996)
1 Introduction
The Constitutive Gene Expression System for Lactococcus lactis and Other Lactic
Acid Bacteria, developed at NIZO food research BV, The Netherlands, is easy-tooperate
and has advantages for the following applications:
Overexpression of homologous and heterologous genes for functional studies and
to obtain large quantities of specific gene products
Metabolic engineering
Suitability for protein secretion (Novotny, R. et al. 2005; Ravn, P. et al. 2003; van
Asseldonk et al., 1990; Vos, P. et al. 1989) and anchoring in the cell envelope
Large scale applications
Major advantages of this system over other expression systems are:
- Less endogenous and no exogenous proteases
- Endotoxin-free expression system
- Food grade protein expression possible
- No formation of inclusion bodies
- No formation of spores
- Simple fermentation, scale-up and downstream processing
- Suitable also for bacteria other than Lactococcus lactis
1.1 Lactococcus lactis
Lactococcus lactis is a homofermentative bacterium. Its primary function is rapid lactic
acid production from lactose. Functional characteristics that have extensively been
studied in lactococci include the carbon metabolism, the extracellular and intracellular
proteolytic system, the production of antibiotic substances, and their interaction with and
resistance to bacteriophages. The genome information of many L. lactis strains is publicly
available. This wealth of knowledge and experience has led to the use of lactococci in
several fields of biotechnology, e.g., the expression of bacterial and viral antigens for
safe vaccination via mucosal immunization, the production of human cytokines and other
therapeutic agents for in situ treatments, the use of lactococci as a cell factory for the
production of specific compounds, and the pilot production of pharmaceutical products
(Mierau, 2005).
1.2 Use of Lactococcus lactis plasmids in other Gram-positive bacteria
Plasmids with a replication origin from L. lactis can be used in all lactic acid bacteria and
in other Gram-positive bacteria such as Bacillus subtilis (Silke David, 1989, AEM
55:1483; Christ Platteeuw, 1994, AEM 60:587; Elisabeth Sorvig, 2005, Microbiology,
151:2439; Indranil Biswas, 2008, Microbiology, 154:2275).
1.3 Codon usage
Until very recently codon usage was an important issue in the possibility and efficiency to
express heterologous genes in L. lactis (GC content of the DNA of 35 - 37%). When a
gene donor organism is closely related to L. lactis, or the DNA GC content is similar to
that of L. lactis, the probability that a gene can successfully be expressed is high. With
the availability of cheap and reliable custom DNA synthesis, there are no longer
restrictions as to the origin of a specific target gene, since, from a known amino acid
sequence, a gene can be designed that fits the codon usage pattern of the host
organism. In addition to a general codon optimization, specific codon tables can be used,
such as the codon table for the highly expressed ribosomal protein genes, to further
increase product formation.